Abstract
Background: Children with sickle cell disease (SCD) are at increased risk of cerebrovascular events that can impact neurocognitive development and quality of life (Colombatti 2016). Transcranial Doppler ultrasound (TCD) is a validated screening tool to identify pediatric SCD patients with the highest risk of stroke to start on a preventive chronic blood transfusion regimen (Estcourt 2020; Inusa 2019). High TCD velocities are an indication to start disease-modifying treatments or consider disease-curative options in children with SCD (Khemani 2019). However, real-world pediatric data on the correlation between hematological variables and TCD results are scarce (Salama 2020). We aimed to evaluate the distribution of TCD velocities in a pediatric natural history cohort and investigate their correlation with hematological variables and treatments.
Methods: We performed a retrospective analysis on data from a prospective pediatric cohort followed from January 1,2009, to December 31, 2020 (censoring date). Standard care includes annual TCD from 2 years of age. We used transcranial Doppler imaging (TCDi) and classified results according to STOP criteria, considering terminal internal carotid artery (TICA) and middle cerebral artery (MCA) time-averaged maximum mean velocities (TAMMVs). Only complete exams with right and left measures available for both vessels were included. Hematological, clinical, and treatment variables were available from the natural history cohort database. Patients were divided according to genotype: HbSS/HbSβ 0 or HbSC/HbSβ +.
Two-sample and Welch t-tests for unequal variances were used to compare mean hemoglobin (Hb) values and hemolysis markers in patients with and without abnormal/conditional TCDi results. Fisher and chi-square tests were used to compare categorical variables. Linear regression models were used to assess the effects of MCA and TICA TAMMVs as continuous variables on Hb. Odds ratios (ORs) for neurological events at different Hb levels were estimated using generalized estimated equations (GEE) with a binomial distribution, logistic function, and exchangeable correlation structure, allowing for correlation among repeated observations for the same patient. Multivariable GEE including characteristics and treatment variables were used to evaluate the association between neurological events and Hb.
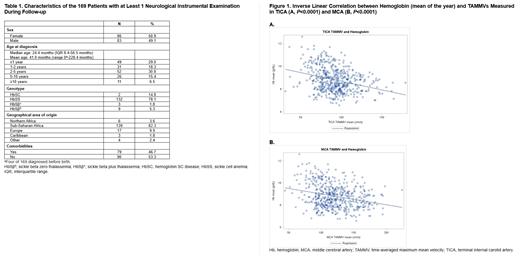
Results: Of the 182 SCD patients in the cohort, 169 had assessments of cerebral vasculopathy, and 155 had evaluable TCDi (583 exams). The median follow-up of the entire cohort was 79.8 months (range: 2.1-298.6 months) (interquartile range [IQR]: 36.9-126.3 months). The median age at the censoring date was 13.4 years (IQR: 9.1-17.5 years); 130 were HbSS/HbSβ 0, and 25 were HbSC/HbSβ +. Basic demographic characteristics of the cohort are in Table 1. The distribution of TCDi results was significantly different between genotypes (P<0.0001): in the HbSC/HbSβ + group (70 exams), 14 were normal (20.0%), and 56 were low (80.0%); in the HbSS/HbSβ 0 group (513 exams), 8 were abnormal (1.6%), 56 were conditional (10.9%), 311 were normal (60.6%), and 138 were low (26.9%). Only 37/138 (26.8%) low TCDi results were confirmed as stenosis at the nearest MRA. Patients with abnormal/conditional TCDi results had lower Hb (8.4 vs 8.9 g/dL, P≤0.0001) and higher reticulocyte counts (317,766 vs 262,750/mm 3, P≤0.0001), lactate dehydrogenase (843 vs 690 U/L, P=0.0012), and aspartate aminotransferase (61 vs 54 U/L, P=0.0007) compared with patients with normal/low results.
We detected a linear correlation between TICA/MCA TAMMVs and Hb (Figure 1A and 1B). Univariate analysis showed significant inverse correlation between abnormal/conditional TCDi results and Hb considered as a continuous variable (OR: 0.484, P<0.001). In the multivariate analysis, the correlation between TCDi results and Hb remained significant; moreover, the risk of presenting abnormal/conditional TCDi results decreased with age (OR: 0.833, P<0.0064).
Conclusions: This analysis from our natural history cohort shows a significant inverse correlation between Hb and MCA and TICA velocities, supporting the beneficial effect of higher Hb levels in reducing TAMMV. Disease-modifying therapies increasing Hb and reducing hemolysis could be helpful in reducing TAMMV in children with SCD.
Funding: This study was supported by Global Blood Therapeutics.
Agodoa: Global Blood Therapeutics: Current Employment, Current equity holder in publicly-traded company. Beaubrun: Global Blood Therapeutics: Current Employment, Current equity holder in publicly-traded company. Biffi: BlueBirdBio: Consultancy, Other: Advisory Board. Colombatti: Global Blood Therapeutics: Research Funding; Addmedica: Consultancy; Forma Therapeutics: Consultancy; Novartis: Consultancy; NovoNordisk: Consultancy; BlueBirdBio: Consultancy; Global Blood Therapeutics: Consultancy; BlueBirdBio: Research Funding.